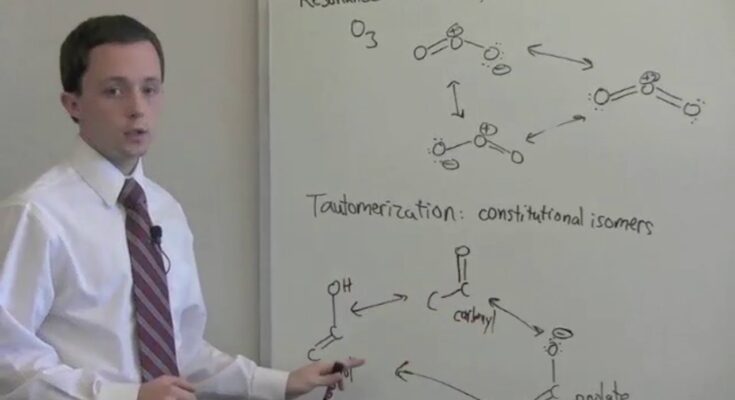
Tautomerism is a phenomenon in which a single chemical compound exists in two or more interconvertible forms with differing relative positions of one atomic nucleus, which is usually hydrogen. The key difference between the two structures, known as tautomers, is the number of electrons and protons. They’re in a condition of dynamic balance with each other.
Desmotropism is another name for tautomerism. Tautomerism is also known by the terms kryptomerism, allotropism, and merotropy. There are various varieties of tautomerism, the most common of which is keto-enol tautomerism. One tautomer occurs as a ketone, whereas the other exists as an enol in this type. We will discuss more on this topic in this article.
Characteristic Features of Tautomerism
Tautomerism has the following characteristics:
- There is a motion of atoms, which are alpha hydrogen atoms.
- Since the compounds are distinct, they may be separated and isolated.
- There are two distinct structures in tautomeric forms.
- Tautomer compounds are in equilibrium with one another.
- The length of the bond is unaffected by tautomerism.
- Tautomerism does not reduce the energy of the molecules, therefore stabilising them.
- It can be found in both planar and non-planar compounds.
Tautomerism’s Structural Requirement
- Polar molecules and weakly acidic functional groups are found in compounds.
- It incorporates the shifting of an atom’s location.
- It has no impact on bond length or other characteristics.
- It can happen in either planar or non-planar molecules.
Types of Tautomerism
Emil Erlenmeyer, a physicist, devised a tautomerism rule in the 1880s. He was one of the first researchers to look at keto-enol Tautomerism. The hydroxyl group in all alcohols is immediately connected to a double-bonded carbon atom, forming aldehydes or ketones, according to this rule. This occurred as a result of the keto form’s increased stability.
Prototropy
The acid-base behaviour of the molecule causes this form of tautomerism. The sole difference between the two types is the location of a proton. The empirical formula and the number of charges will be the same in this arrangement.
Annular Tautomerism
An annular tautomerism is a process in which a proton occupies two or more places in a heterocyclic system. If an open structure is changed to a ring structure due to the delocalisation of protons in tautomerism, such a tautomer is known as a ring-chain tautomer. Glucose is an example of a ring-chain tautomer.
Valence tautomerism
It is a kind of tautomerism in which single and double bonds are continuously formed and broken in the compound without any group or atom movement. It differs from the preceding form of tautomerism in that it occurs quickly.
- There is a shift in geometrical structure in this tautomerism, but no change in canonical resonance structure or mesomers.
Examples
Tautomerism is defined as a kind of isomerism in which the isomers easily swap into/between one another, allowing them to coexist in equilibrium. A proton transfer happens in an intramolecular manner during the process. Consider the following tautomerism example. Tautomers include ketone-enol, enamine and imine, and lactam-lactim, to name a few.
- A chemical equilibrium between the keto form (a carbonyl structure containing -hydrogen) and the enol form (a double bond next to alcohol, -C=C-OH) of a substance is known as keto-enol tautomerism.
- The nitrogen counterpart of keto-enol tautomerism is imine-enamine tautomerism. A hydrogen atom trades places with the heteroatom (oxygen or nitrogen) and the second carbon atom in both instances.
Summary
- Tautomerism is the phenomenon of a single chemical molecule tending to exist in two or more interconvertible forms that differ in terms of the location of one atomic nucleus, which is usually hydrogen.
- The number of electrons and protons in the tautomers differs.
- Protons are transferred when a reaction between tautomers occurs.
- Tautomerism allows isomers to easily swap into or between one another in order to coexist in equilibrium.
- Proton transfer takes place between molecules during the process.